Germ Cell Tumors in Gonads: Navigating Traditional Pathways, Venturing into Uncharted Territories, Pushing the Frontiers.
- sunshine4cancerkid
- Aug 15, 2024
- 38 min read
Updated: Oct 11, 2024
Alice, Ellen, Jasleen, Rakshitha
Pediatric Cancer Research Writers Program
Discussion
Exploring the study of gonadal germ cell tumors was an fascinating experience allowing our team to discover new insights on an obscure subject. This study led to the introduction of the complexity and consequences of gonadal germ cell tumors. A prominent aspect was the intricacy of their development and differentiation between genders and various factors that are unanticipated. This analysis stretched our insight and awareness on GCTs and triggered curiosity of the connection between medical professions, developmental aspects and the complexity of GCTs.
Abstract
Gonadal germ cell tumors (GCTs) present significant diagnostic and therapeutic challenges due to their diverse histological types and biological behaviors. This study reviews the various types of GCTs, focusing on the differences between seminomas and non-seminomatous germ cell tumors (NSGCTs), and their occurrence in the testes and ovaries. Seminomas, including testicular seminomas and ovarian dysgerminomas, are characterized by uniform cells and generally have a favorable prognosis with radiation and chemotherapy. NSGCTs, comprising embryonal carcinoma, yolk sac tumor, choriocarcinoma, and teratomas, are more aggressive and often require complex treatment strategies. Teratomas are particularly notable for their histological diversity, with mature teratomas being benign in children and women but potentially malignant in postpubertal males. The study also highlights mixed germ cell tumors, which combine various GCT components, complicating diagnosis and treatment.
This review underscores the importance of immunohistochemical markers such as PLAP, CD117, and OCT3/4 in differentiating GCT types and guiding treatment. Additionally, the unique features of spermatocytic seminomas and the phenomenon of 'burnt-out' germ cell tumors are discussed, emphasizing the distinct pathophysiological mechanisms in the testes. The findings suggest that understanding the histopathological and molecular characteristics of GCTs is crucial for accurate diagnosis, prognosis, and personalized therapeutic approaches.
Keywords: Gonadal germ cell tumors, seminomas, non-seminomatous germ cell tumors, teratomas, immunohistochemical markers, testicular cancer, ovarian cancer, embryonal carcinoma, yolk sac tumor, choriocarcinoma.
Introduction
Gonadal germ cell tumors (GCTs) are a diverse group of neoplasms that arise from germ cells within the gonads, namely the testes in males and the ovaries in females. These tumors encompass a broad spectrum of histological types, each exhibiting unique biological behaviors and clinical outcomes. GCTs are particularly intriguing from an academic and clinical perspective due to their varied presentation in the two gonads and the significant differences in frequency and nature of specific neoplasms within each.
The classification of GCTs broadly divides them into seminomas and non-seminomatous germ cell tumors (NSGCTs). Seminomas, including testicular seminomas and ovarian dysgerminomas, are characterized by relatively uniform cells and generally present with a favorable prognosis when treated with radiation and chemotherapy. In contrast, NSGCTs, which include embryonal carcinoma, yolk sac tumor, choriocarcinoma, and teratomas, are typically more aggressive and often necessitate a combination of surgical intervention and multi-agent chemotherapy.

Diagnostic challenges in GCTs are compounded by their ability to mimic other neoplasms, necessitating the use of specific immunohistochemical markers such as placental alkaline phosphatase (PLAP), CD117, and OCT3/4 to accurately differentiate between tumor types. Unique variants, such as spermatocytic seminomas which occur exclusively in the testes, and the phenomenon of 'burnt-out' germ cell tumors, add to the complexity of diagnosis and highlight the distinctive pathophysiological mechanisms at play within the testes.
Therefore, this article provides a detailed and updated overview of the literature regarding the occurrence of germ cell tumors in patients and outlines the major recent progress that has been made in our understanding of the pathogenesis of germ cell tumors and the early recognition of (pre-)neoplastic changes.
Classification of Testicular GCTs:
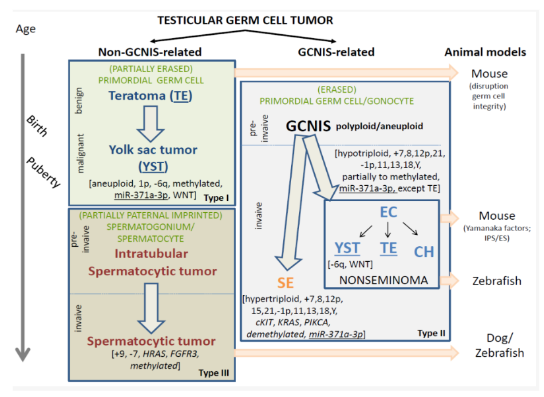
The types of testicular GCTs are divided into two main categories. The Type I and III testicular GCTs are together referred to as non-GCNIS-related GCTs. The Type II testicular GCTs are referred to as GCNIS-related GCTs. This is simply based on the recognition of the different cells of origin and related pathogenesis, in which the knowledge on the origin of the Type II tumors (i.e., GCNIS, see below), is the dominant player in the classification because of its well-recognized status. This distinction between the GCNIS-related and non-GCNIS-related testicular GCTs is of relevance because of their different clinical behavior, i.e., malignant versus (predominantly) benign. As such, they will be discussed separately. Of special notion is the fact that, so far, morphology, mRNA, microRNA, and protein profiles of (Type I and II) teratoma and yolk sac tumor elements are similar; therefore, noninformative to make a differential diagnosis. However, this is consistently the case regarding their molecular genetic make-up, being therefore of diagnostic value. In addition, various animal models have been reported to be informative for GCT, which will be summarized hereunder because of their potential impact in understanding the pathogenesis of this type of cancer.
.
Figure 1. Schematic representation of the various entities of testicular germ cell tumors (GCTs). The timeline is indicated on the left side and the proposed animal models on the right. The GCTs include the non-GCNIS (germ cell neoplasia in situ) related GCTs (left panel) and GCNIS-related GCTs (right panel). The non-GCNIS related GCTs are subcategorized into the prepubertal teratomas (TE) and yolk sac tumors (YST) as well as the spermatocytic tumors. These are also referred to as Type I and III, respectively. The GCNIS-related GCTs are histologically (and clinically) subdivided into the seminomas (SE) and the various elements of nonseminomatous GCTs, being embryonal carcinoma (EC), YST, choriocarcinoma, and TE. Note the overlapping histology between the prepubertal TE/YST and the TE and YST elements in the GCNIS-related nonseminomas.
However, they have a separate (and independent) pathogenesis. The presumed cells of origin are indicated in green, reflecting a (partially and fully erased) primordial germ cell (Type I and II), to partially paternal imprinted spermatogonium/spermatocyte (Type III). The precursors are indicated when known (preinvasive), while specifically the benign and malignant behavior of the pediatric TE and YST is highlighted. In addition, the most prominent and recurrent molecular genetic changes are indicated, of putative interest to be used for molecular pathological approaches. These include total genomic anomalies, like polyploid/aneuploid, specific chromosomal imbalances like losses (-) and gains (+), as well as recurrent mutations (italics). In addition, the methylation status is indicated as well as the possible use of miR-371a-3p as a liquid biopsy molecular biomarker (underlined). All malignant histological elements, independent of age, are identified by this biomarker (except TE). The WNT pathway is specifically involved in the YST components, independent of age and also of pathogenesis.
Arrested germ cell development is an important step in the development of TGCTs. Because the pathogenesis of GCNIS-related TGCTs starts before birth, understanding normal germ cell development is imperative to comprehending the pathogenesis of TGCTs. The gonocytes or primitive germ cells with stem cell–like markers such as receptor tyrosine kinase (c-KIT )and octamer binding transcription factors 3 and 4 (OCT3/4) may be identified at the bilaminar disk stage of the embryo at approximately 2 weeks of gestation. Primitive germ cells colonize the testicular parenchyma after extensive migration and, by puberty, lose the primitive markers to transform into mature sex cell progenitors. During migration, there is a hypothetical risk of gonocytes being deposited along the pathway, leading to the development of extragonadal GCTs. Because of activation of the hypothalamic-pituitary-gonadal axis at puberty, the gonocytes enter meiosis, triggered by the loss of expression of DMRT1 (sex-determining transcription factor), and obtain a haploid genotype.
Classification of Ovarian GCTs:
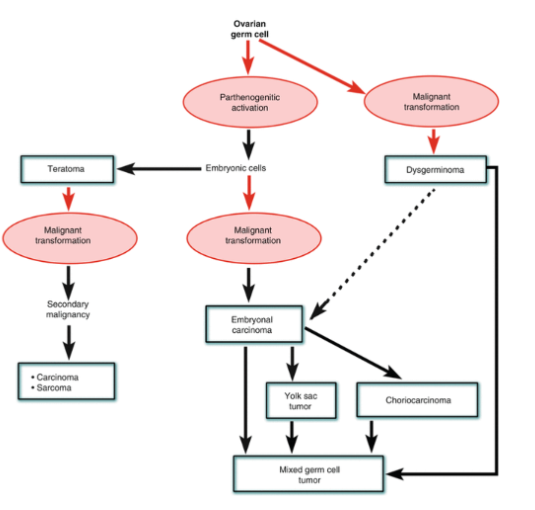
Ovarian germ cell tumors are basically equivalent to those originating from male germ cells. Yet there are some important biological and clinical differences between these two groups of tumors . For example, in contrast to the malignant nature of the vast majority of testicular tumors, most ovarian tumors are benign, presenting clinically as mature teratomas.Experimental data obtained in mice indicate that ovarian teratomas are formed from parthenogenetically activated ovarian germ cells. Human parthenotes isolated from the ovaries can rise to embryonic stem cells, and thus by extrapolation, one can assume that these cells could give rise to teratomas and other germ cell tumors as well.
The histogenesis of teratoma can be readily explained by parthenogenetic activation of ovarian germ cells. The histogenesis of malignant ovarian germ cell tumors is a bit more complicated, and several histogenetic schemes have been proposed, as reviewed by J. Prat in the monograph which he has edited with G. Mutter . Despite many attempts to modify our understanding of malignant ovarian germ cell, histogenesis is still incomplete. The panel of experts of WHO has thus decided to base the latest WHO classification of malignant germ cell tumors on the most popular model of histogenesis of these tumors dating back to the work of Teilum. According to this scheme, the malignant germ cell can form either dysgerminoma or embryonal carcinomas, which in turn could give rise to choriocarcinoma, yolk sac carcinoma, or teratoid tumors.
Figure 2. Schematic representation of the various entities of ovarian germ cell tumors (GCTs). Ovarian germ cell tumors are classified into several subtypes, each with distinct characteristics: Dysgerminomas are the most common malignant type, typically found in younger women and highly responsive to treatment. Yolk sac tumors are aggressive malignancies that secrete alpha-fetoprotein (AFP) and require prompt chemotherapy. Teratomas can be benign (mature teratomas) or malignant (immature teratomas), with the benign form being the most common ovarian germ cell tumor overall. Choriocarcinomas are rare, highly malignant tumors associated with elevated human chorionic gonadotropin (hCG) levels. Embryonal carcinomas are rare, aggressive tumors that often coexist with other germ cell tumor types.
In the expanded histogenetic algorithm presented above, we propose that the tumor formation depends in all cases on parthenogenetic activation of the ovarian germ cells [ova], which may give rise as such to benign tumors, i.e., teratomas. Alternatively, if the germ cells undergo malignant transformation, they may give rise to dysgerminoma or form embryonal carcinoma cells. EC cells may form either a monotypic tumor-embryonal carcinoma or by differentiating into somatic and extrasomatic cells and tissues form the malignant stem cells of a malignant mixed germ cell tumor. Even monotypic EC tumors contain syncytiotrophoblastic cells, which may be present in most of such tumors. EC cells can sometimes differentiate into yolk sac or choriocarcinoma cells, which as such may form tumors of the same name, overgrowing the EC component, which may be hard to find. Alternatively, EC cells may remain part of the mixed germ cell tumors which in such cases will contain several other distinct components: EC cells, teratomatous tissues, yolk sac tumor, and choriocarcinoma, or various combinations of these elements. There is also some evidence that dysgerminomas may give rise to embryonal carcinoma, but there is no doubt that they can be part of mixed germ cell tumors.
Diagnosis
Diagnosis of GCTs requires numerous complex steps to accurately discern the subtype and stage of the tumors. The multimodal approach ensures a comprehensive assessment of the nature of the tumor, guiding both the diagnosis and subsequent treatment of GCTs.
First, clinical presentation of symptoms similar to those produced by gonadal germ cell tumors is required to consider a potential diagnosis of GCTs. Common GCT symptoms include painful or painless testicular masses, a solid ovarian mass, and a mass visible in the lower back near the anus, often in an infant. Development of pubic hair, breast enlargement, or vaginal bleeding at a very young age may also be a sign of GCTs. Extragonadal germ cell tumors, a rarer type of GCTs, exhibit symptoms that vary widely depending on the location. These symptoms may include chest pain, cough, dyspnea (in cases of mediastinal tumors), or abdominal pain (in cases of retroperitoneal tumors). In advanced cases, both GCT and extragonadal GCT symptoms may include abdominal or back pain, gynecomastia, or respiratory distress associated with a mass inside the chest if metastasis has occurred.
If there is an adequate clinical presentation of GCT symptoms, medical professionals will perform a careful history examination of the patient. Health history can sometimes indicate a higher risk for GCTs; for example, those born with abnormal ovaries or testes due to genetic conditions such as Turner’s or Klinefelter’s are more susceptible to GCT growth. A thorough physical examination is performed after, either in the testicular area for males or the pelvic area for females. If the medical professional suspects the presence of an extragonadal GCT, a thorough palpation of the abdomen, supraclavicular regions, and chest is warranted.
Oftentimes, these examinations reveal firm, non-tender masses or an adnexal mass. After physical examinations, a complete diagnostic evaluation is obtained through various imaging modalities, laboratory studies, and surgical resections. Imaging modalities include an ultrasound of the testes and the ovaries as well as any possible masses, which provides detailed information on the potential GCTs; computed tomography (CT) scan of chest, abdomen, and pelvis, typically employed to assess the extent of GCTs and detect metastasis; and an X-Ray to form an image of the abdomen or the chest, where extragonadal tumors tend to grow. Medical professionals then perform blood tests to measure blood counts and liver and kidney function, properly assessing the health of the patient. Additional blood tests to monitor serum tumor marker levels may also be ordered. Serum tumor markers, such as Alpha-fetoprotein (AFP) and Human Chorionic Gonadotropin (hCG), play a critical role in the diagnosis, prognosis, and monitoring of GCTs because their levels are elevated in some types.
Finally, definitive diagnosis requires histological examination of tumor tissue. This is achieved through surgical resections and biopsies. The type of biopsy performed depends on the location and size of the tumor; with fine-needle aspiration, tissue is removed with a thin needle, while the entire tumor is removed in surgical biopsies. Usually, ovarian or testicular GCTs are removed along with the involved testes or ovary. Abdominal lymph nodes and extragonadal GCTs tumors arising in the lower back, the chest, or elsewhere will be removed surgically, if possible, or biopsied. The tissue is then analyzed carefully.
Depending on the pathology, GCTs are sorted into different subtypes. A GCT can be classified as a benign teratoma, malignant teratoma, yolk sac tumor (high AFP test), choriocarcinoma (high bHCG test), embryonal carcinoma, and germinoma. GCTs are then staged based on the tumor's size, presence of metastasis, and serum tumor marker levels. The stages, ranging from Stage I to Stage IV, determine the condition of the GCT and the treatment plan necessary for the patient. Stage I refers to a tumor that was completely resected. Stage II tumors are resected but leave a few cells behind. Stage III tumors have spread to the lymph nodes, while Stage IV tumors have spread to the lung, liver, or other locations. After diagnosis and staging, patients with GCTs undergo treatment immediately.
Imaging
In-Situ GCT’S:
GCNIS is found in the testicular parenchyma adjacent to TGCTs in 90% of TGCTs and in the contralateral testis in 4%–8% of TGCTs. Patients with cryptorchidism or atrophic testis are at higher risk for GCNIS and TGCTs. Approximately 70% of GCNIS-positive men may develop TGCT within the next 7 years. Lenz et al (37) showed that an irregular or coarse echogenic appearance of the testicular parenchyma at US may suggest the possibility of GCNIS. However, this finding has not been confirmed in larger studies. The positive predictive value is low (22%). Currently, US has no role in screening for GCNIS.
1. Seminomas:
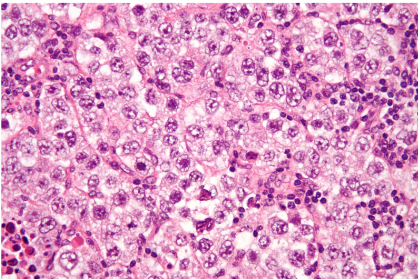
Pure seminomas, which account for up to 50% of TGCTs, commonly occur in men aged 30–40 years (38,39). Serum lactate dehydrogenase levels are elevated in patients with seminomas, and approximately 15% of patients may have a mildly elevated β-hCG level because of the presence of syncytiotrophoblastic cells. At pathologic examination, seminomas are usually solid tumors with a pale tan slightly lobulated cut surface. At histologic examination, a diffuse arrangement of large polygonal cells that are divided into large lobules by fibrous bands is a typical finding. In immunohistochemical analysis, seminoma cells stain positive for c-KIT, placental alkaline phosphatase, and OCT3/4 .
The imaging appearance reflects the uniform cellular composition of seminomas. At US, seminomas typically are homogeneous, hypoechoic, and solid appearing, with well-circumscribed or lobulated margins, and they show increased vascularity along the fibrovascular septa at Doppler US. At MRI, seminomas are homogeneously T1 hypointense and T2 hypointense, with well-circumscribed or lobulated margins. At diffusion-weighted MRI, marked restricted diffusion secondary to increased cellularity and underlying inflammation can be seen; bandlike hyperenhancement of fibrovascular septa is characteristic of GCNIS
.
Figure 3. Pure seminoma in a 35-year-old man. (A) Photograph of the sectioned gross specimen demonstrates a testicular mass with a pale tan lobulated cut surface (arrows). (B) Photomicrograph shows nests of large polygonal cells with moderate eosinophilic cytoplasms and prominent nucleoli (arrows) that are separated by fibrous bands containing a lymphocytic infiltrate (arrowheads), which are consistent with a pure seminoma. (Hematoxylin-eosin stain; original magnification, ×20.) (C, D) Gray-scale (C) and color Doppler (D)US images of the scrotum show a well-circumscribed lobulated homogeneously hypoechoic testicular mass (arrow) with increased vascularity.
2. Non-Seminomatous germ cell tumors:
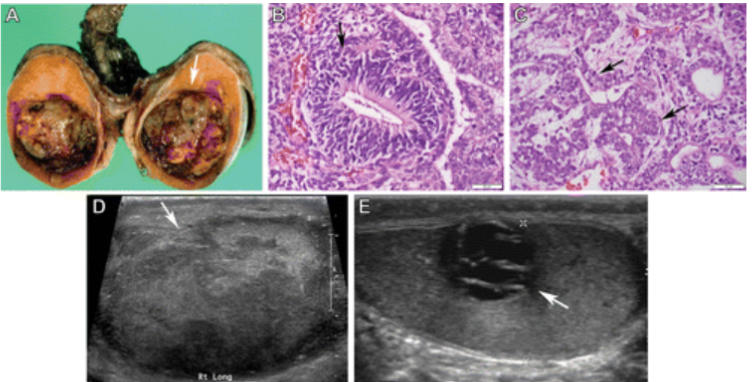
NSGCTs comprise 30%–40% of all TGCTs and may be pure or mixed tumors. Mixed tumors show variable proportions of embryonal carcinoma, yolk sac tumors, postpubertal teratomas, and choriocarcinomas. NSGCTs are diagnosed in men during the 3rd decade of life. Unlike seminomas, NSGCTs manifest with advanced disease in 60% of patients. The prognosis is unfavorable for tumors with a higher clinical stage. Imaging findings of NSGCTs reflect their variegated nature and the proportion of specific histologic types. At US, NSGCTs are typically heterogeneous; have ill-defined margins; and contain cystic areas and echogenic foci that correspond to necrosis, hemorrhage, and calcifications. Cysts within NSGCTs correspond to epithelium-lined true cysts in teratomas and dilated rete testis or necrosis in other tumors. The presence of solid hypoechoic areas in NSGCTs commonly corresponds to embryonal carcinoma and yolk sac tumor components, whereas hemorrhagic areas or metastases with hemorrhage indicate choriocarcinoma . NSGCTs show heterogeneous signal intensity at T1- and T2-weighted MRI, with hemorrhage and necrosis. NSGCTs also show heterogeneous contrast enhancement and frequent invasion of adjacent structures.
Figure 4. NSGCTs in three patients. (A–C) Pathologic findings of an NSGCT with a predominant yolk sac tumor and embryonal carcinoma in a 26-year-old man. Photograph of the sectioned gross specimen (A) shows a discrete solid-cystic mass with areas of hemorrhage and necrosis (arrow in A). Photomicrograph of the yolk sac tumor component (B) shows tumor cells palisading around a central capillary, known as a Schiller-Duval body (arrow in B). (Hematoxylin-eosin stain; original magnification, ×40.) Photomicrograph of the embryonal carcinoma component (C) shows a tubulopapillary appearance of cytologically anaplastic-appearing cells (arrows in C). (Hematoxylin-eosin stain; original magnification, ×40.) (D) Gray-scale US image of the right testicle in a 22-year-old man with a pathologically proven NSGCT (mostly a mixture of embryonal carcinoma and postpubertal teratoma) shows an ill-defined heterogeneously hypoechoic mass (arrow) replacing the entire testicular parenchyma. (E) Gray-scale US image of the left testicle in a 24-year-old man with a pathologically proven NSGCT (99% teratoma) shows a cystic mass (arrow) with thickened septa.
3. Embryonal carcinomas:
Aggressive features such as extratesticular extension and metastatic disease are common in TGCTs that show embryonal carcinoma. At histopathologic examination, anaplastic-appearing cells with diverse architectural patterns are seen.
At US, embryonal carcinoma is a solid predominantly hypoechoic heterogeneous mass with ill-defined margins, contour abnormality of the testicle, and invasion of the tunica albuginea. Ill-defined hypoechoic areas and echogenic foci represent hemorrhage, necrosis, and calcifications. At MRI, the tumor shows heterogeneous signal intensity, with areas of necrosis and hemorrhage.
Figure 5. NSGCT with a predominant embryonal carcinoma component in a 24-year-old man. Gray-scale US image of the scrotum shows a lobulated heterogeneously hypoechoic testicular mass (arrow) with invasion of the adjacent tunica albuginea (arrowhead). This mass was proved to be an NSGCT with 95% embryonal carcinoma and 5% postpubertal teratoma at pathologic examination.
4. Post Pubertal Yolk-Sac Tumor:
A pure postpubertal yolk sac tumor in adults is rare and is almost always part of an NSGCT. The tumor demonstrates extraembryonic differentiation into yolk sac elements and is commonly associated with marked elevation of serum α-fetoprotein levels. At pathologic examination of gross specimens, the yolk sac tumor component in an NSGCT appears as a solid to cystic area with a gray to tan myxoid cut surface and foci of necrosis and hemorrhage. Tumor cells are positive for glypican-3 and α-fetoprotein at immunohistochemical analysis.
At imaging, NSGCTs with a predominant yolk sac tumor component may manifest as ill-defined masses with varying amounts of cystic change, indicating necrosis and echogenic foci that correspond to hemorrhage. The tumor usually demonstrates variable signal intensity at T1- and T2-weighted MRI because of hemorrhage and cystic degeneration.
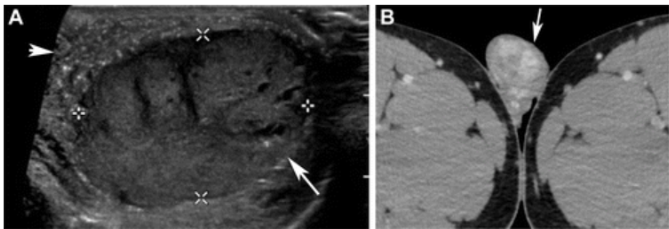
Figure 6. NSGCT with a predominant yolk sac tumor component in a 19-year-old man. (A) Gray-scale US image of the scrotum shows a well-circumscribed hypoechoic testicular mass with scattered cystic areas (arrow) and TM in the remaining testicle (arrowhead). (B) Axial contrast-enhanced CT image of the scrotum shows a well-circumscribed heterogeneously enhancing mass in the right testicle (arrow). This mass was proved to be an NSGCT with 99% postpubertal yolk sac tumor.
5. Choriocarcinoma:
Up to 16% of NSGCTs show choriocarcinoma, which is the most aggressive TGCT. The pure form of this tumor is rare. Choriocarcinoma is associated with microscopic vascular invasion, resulting in early widespread hematogenous dissemination. Serum β-hCG levels are markedly elevated. The presence of hemorrhagic nodules is typical at pathologic examination of gross specimens. Extensive areas of hemorrhage that are surrounded by a mixture of trophoblasts with vascular invasion are seen at histopathologic examination. Tumor cells are positive for β-hCG and GATA binding protein 3 (GATA3) at immunohistochemical analysis.
Figure 7. (A, B) Testicular choriocarcinoma in a 29-year-old man. (A) Photograph of pathologic specimens shows a hemorrhagic tumor rimmed by a gray-tan area (arrows). (B) Photomicrograph shows large multinucleated syncytiotrophoblasts and intermediate-sized cytotrophoblasts (arrows), with areas of hemorrhage (arrowheads). (Hematoxylin-eosin stain; original magnification, ×20.) (C, D) Choriocarcinoma in a 39-year-old man. Gray-scale US image of the scrotum (C) shows an ill-defined heterogeneously hypoechoic mass with cystic areas (arrows in C) that represent hemorrhage and necrosis.
6. Postpubertal Teratoma:
A pure teratoma is uncommon in postpubertal male patients, and most tumors manifest as a component of an NSGCT. Serum tumor marker levels are normal. One-third of patients present with advanced disease, including mediastinal or intracranial masses, which are associated with a higher incidence of recurrence. At pathologic examination of gross specimens, tumors are often solid to cystic masses, with histologic features that vary depending on the tissue types present.
Imaging shows predominantly cystic lesions with macroscopic fat, calcifications, and solid components, depending on their immaturity. At US, these masses are markedly heterogeneous and predominantly cystic. Echogenic areas may correspond to calcification, cartilage, immature bone, or macroscopic fat. At MRI, teratomas are well-circumscribed complex cystic masses with signal intensity that varies according to the contents of the tumor (eg, serum, mucin, or keratin). Somatic-type malignancies that arise in the teratoma (ie, previously called teratocarcinomas) are seen in approximately 3%–6% of teratomas. They may arise in the testes or retroperitoneal lymph nodes and are associated with a poor prognosis. Sarcoma is the most common somatic-type malignancy, the majority of which are rhabdomyosarcomas.
Figure 8. Pure postpubertal teratoma in a 42-year-old man. (A) Gray-scale US image of the scrotum shows a mixed solid and cystic testicular mass with a few punctate echogenic foci (arrow). (B) Axial contrast-enhanced CT image of the abdomen shows a retroperitoneal lymph node with multiple calcifications (arrowhead). Postpubertal teratoma was diagnosed on the basis of pathologic examination.
7. Burned-Out TGCTs:
A burned-out TGCT is a metastatic TGCT in which the primary tumor has completely or partially regressed, forming a fibrotic scar-like area. Burned-out TGCTs manifest exclusively by means of metastasis, most commonly in the retroperitoneum. Proposed mechanisms include ischemic regression from rapid tumor growth that exceeds perfusion and immune-mediated regression . The most frequent subtypes that regress are NSGCTs that predominantly contain teratomas or choriocarcinomas. At pathologic examination, the regressed areas show dense fibrosis with scar formation, with or without tubular lithiasis, and lymphoplasmacytic inflammation.
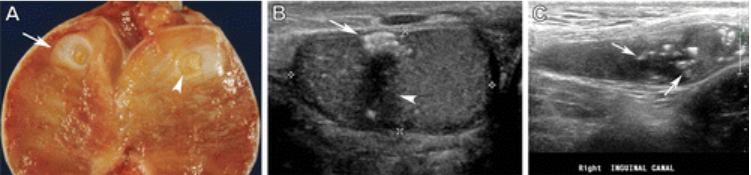
Figure 9. Burned-out TGCT in three patients. (A) Photograph of the sectioned gross specimen in a young man (in his 20s) shows the testicular parenchyma with a fibrotic scar like area (arrow) and central calcification (arrowhead). (B) Gray-scale US image of the scrotum in a 26-year-old man shows a focus of hyperechoic calcification (arrow) and an ill-defined hypoechoic area (arrowhead) with predominant choriocarcinoma components. (C) Gray-scale US image of the right inguinal canal in a 17-year-old adolescent boy shows an atrophic and undescended testicle with multiple hyperechoic calcifications (arrows).
Ex-Situ GCT’s:
1. Prepubertal Teratoma and Associated Tumors:
Prepubertal teratomas and associated tumors account for 65% of testicular tumors in prepubertal children. They have an excellent prognosis compared with that of postpubertal teratomas. Dermoid cysts, epidermoid cysts, and well-differentiated neuroendocrine tumors are distinct types of prepubertal teratomas. These tumors are also seen in adults, especially epidermoid cysts. A prepubertal teratoma can be mature or immature. Younger patients and those with larger tumors and elevated α-fetoprotein levels are more likely to have immature teratomas. At US, mature teratomas commonly manifest as cystic lesions that contain sebaceous material that contributes to a heterogeneously echogenic appearance. Immature teratomas are typically larger and solid from neuroectodermal components. Imaging features may also change over time. CT and MRI can show fat, calcification, and cystic components in mature teratomas.
2. Prepubertal Yolk Sac Tumors:
A pure form of prepubertal yolk sac tumor is the most common testicular malignancy in children younger than 2 years. Elevated serum α-fetoprotein levels are seen and used for diagnosis and surveillance. Tumors are histologically similar to the postpubertal type, except for a lack of GCNIS and the absence of regression.
At US, the prepubertal yolk sac tumor manifests as either a homogeneous solid hypoechoic mass that can involve the entire testicular parenchyma or as a heterogeneous mass with solid and cystic areas. At MRI, the mass is T2 hyperintense and T1 hypointense, with restricted diffusion and heterogeneous contrast enhancement.
3. Spermatocytic Tumors:
A spermatocytic tumor is a rare TGCT (ie, it represents only 1% of testicular cancers) that is derived from mature germ cells such as a spermatogonium or an early spermatocyte. These tumors rarely metastasize, and patients with them have normal serum tumor marker levels. In contradistinction to a seminoma, this tumor manifests in men during the 6th decade of life. At imaging, a spermatocytic tumor appears as a large (>5 cm) well-defined multinodular heterogeneous mass with cystic foci. It has an excellent prognosis, without recurrence or metastasis.
Medical Professions
The management of gonadal germ cell tumors (GCTs), which develop in the testes or ovaries, necessitates the involvement of a specialized team of healthcare professionals responsible for diagnosis, treatment, and ongoing care. This team typically includes a diverse range of medical experts with distinct roles and responsibilities in addressing the complexities of these types of cancers.
1.Medical Oncologist:
These dedicated professionals coordinate all aspects of a patient's cancer treatment plan. Their job involves diagnosing various cancers, discussing treatment options with patients, arranging and supervising drug treatments, and drafting chemotherapy regimens tailored for GCTs. They also expertly manage any treatment-related complications and provide unwavering patient support while overseeing their care. Their extensive training involves four years of medical school, three years in an internal medicine residency, and a two-year medical oncology fellowship, totaling nine years after completing a four-year undergraduate program, also known as pre-med. Salaries vary widely based on location, experience, and employer. The average salary for medical oncologists in the United States is $457,516 per year, with an average additional cash compensation for a Medical Oncologist in the United States is $216,441, with a range from $162,331 - $303,017.
2.Surgical Oncologist:
Surgical oncologists are central in managing gonadal germ cell tumors (GCTs). They perform surgeries that provide tissue for initial diagnosis and staging but aim for a complete cure for early-stage disease. In advanced cases, surgery can debulk tumors before other treatments or assess response to chemotherapy. They are skilled in conducting critical procedures such as orchidectomy (testicle removal) or hysterectomy (uterus removal), depending on the patient's gender. To become a surgical oncologist, one must complete four years in medical school, a 5-year general surgery residency, and 2-3 years of surgical oncology fellowship, totaling 11-12 years after completing a 4-year undergraduate program, also known as pre-med. The average surgical oncologist salary in the United States is $284,293. Surgical oncologist salaries typically range between $146,000 and $552,000 yearly.
3.Urologists:
They are the primary specialists in managing gonadal germ cell cancer (GCTs) due to their expertise in the male reproductive system. They diagnose the cancer through physical exams, imaging studies, and biopsies, and perform surgeries like orchidectomy and lymph node dissection. Urologists also collaborate with medical oncologists for chemotherapy and other treatments, monitor patients for recurrence, and provide long-term care, including fertility management. To be a urologist, you will need four years of medical school, 4-5 years of urology residency, and an extra 1 or 2 years of fellowship if going into more specialized areas, totaling 9-11 years of schooling after receiving a college degree. The average Urologist salary in the United States is $423,360, but the range typically falls between $371,530 and $493,760. Salary ranges can vary widely depending on many important factors, including education, certifications, additional skills, and the years you have spent in your profession.
4.Gynecologists:
In women, a collaborative approach involving gynecologists, medical oncologists, and surgical oncologists is common. Gynecologists play a big part in diagnosing and treating ovarian germ cell tumors (GCTs), preserving fertility, and managing hormone imbalances associated with these cancers. Future gynecologists will have to go through 4 years of medical school, followed by four years of obstetric gynecology residency and an extra three years if going into any specialty, for example, maternal-fetal medicine or gynecologic oncology. In the United States, the average salary for a Gynecologist is $320,000, with a typical range between $277,600 and $385,000. The salary range can vary widely based on education, certifications, additional skills, and years of experience.
5.Radiology oncologist:
A radiation oncologist specializes in treating cancer with radiation therapy. These specialists use radiation therapy to treat specific types of germ cell tumors (GCTs), especially those resistant to chemotherapy. Radiation oncologists have experience in treating all types of cancer using various forms of radiation. ]They must complete four years of medical school, one year of clinical training in internal medicine or surgery, and four years of residency in radiation oncology. In the United States, the average annual salary for a Radiation Oncologist is $460,200. However, the salary range can vary widely, falling between $396,560 and $528,400. This wide range is influenced by education, certifications, additional skills, and years of experience.
6.Pathologists:
These doctors play a critical role in confirming the diagnosis by carefully examining tissue samples, identifying the specific type of germ cell tumor (GCT), and accurately staging the cancer. To be a pathologist, you will need to complete four years of medical school, 3-4 years of pathology residency, and an optional two years of fellowship if you want to go into a specialty under pathology, totaling to 9-10 years of schooling after finishing a pre-med program (undergraduate degree). The average annual pay for a Pathologist in the United States is $337,500. The majority of pathologist salaries currently range between $285,000 to $378,500 across the United States.
7.Pediatric oncologists:
They are vital in treating germ cell tumors (GCTs) in children and adolescents. They provide essential support to both young patients and their families throughout their cancer treatment. Pediatric oncologists are responsible for administering chemotherapy, which can be delivered through an IV infusion, in a pill or liquid, or via injection. Their years of schooling include four years of medical school, three years of pediatric residency, and three years of pediatric oncologist fellowship, totaling ten years of education after receiving an undergraduate degree, also known as the pre-med. The average annual salary for a Pediatric Oncologist in the United States is $175,055, with the current salary range falling between $170,000 and $217,500.Although many other specialists work with germ cell tumors (GCTs), these are the main professionals involved. An endocrinologist or radiologist may be added depending on the patient's case.
Treatments
GCTs are generally treated with chemotherapy, a drug therapy that uses chemicals to kill fast-growing cells in the body. However, specific treatment plans depend on the tumor’s stage and location. For Stage 1 GCTs located in the testes or ovaries, no chemotherapy is required unless another GCT forms. For Stage II-IV GCTs in the testes, Stage II-III GCTs in the ovary, and Stage I-II non-testes or ovary GCTs, patients are treated with cisplatin, etoposide, and bleomycin (anti-cancer drugs) intravenously every 3 weeks for 3 cycles. For Stage IV tumors in the ovary and Stage III-IV non-testes or ovary GCTs, cisplatin, etoposide, and bleomycin are administered intravenously every 3 weeks for 4 cycles. In some instances, radiation therapy is used instead of chemotherapy for germinoma. If there is a residual tumor present after initial treatment, additional surgical resection and chemotherapy may be necessary.
Sometimes, patients experience relapses of GCTs. Stage I patients who relapse after surgical resection alone are treated with 3-4 cycles of cisplatin, etoposide, and bleomycin chemotherapy. Patients suffering with other staged tumors who relapse after receiving chemotherapy are treated with additional chemotherapeutic drugs such as Paclitaxel, Ifosfamide and Carboplatin as well as autologous stem cell transportation. Autologous stem cell transplantation describes the process of harvesting the patient’s blood or marrow stem cells, treating the patient with very high doses of carboplatin and etoposide, and re-infusing the patient’s own stem cells. “Autologous” refers to the use of the patient’s own stem cells to avoid immune rejections and other risks of receiving stem cells from donors. This transplantation is used to mitigate the negative effects of intense chemotherapy; the high doses of carboplatin and etoposide not only destroy cancer cells but also the bone marrow, where blood cells are produced. Therefore, the patient’s stem cells need to be collected before chemotherapy treatment and reinfused back into the bloodstream afterwards. These stem cells travel to the bone marrow, where they begin to produce new and healthy blood cells. Tandem transplantation, which refers to when this procedure is done twice, is used in more severe cases of GCTs.
Late relapses (GCTs that occur more than 2 years after initial chemotherapy) in seminomas are given the same treatment as early relapses in seminomas (chemotherapy alone). However, for late relapses in nonseminomatous GCTs, chemotherapy alone is rarely curative and the prognosis for such patients is much worse. As a result, a combination of surgery and chemotherapy is used to improve cure rates.
Once treatment is complete, patients are followed for recurrence through physical examinations, blood tests for AFP or bHCG (if they were abnormal initially), CT scans of the chest and abdomen, and chest X-rays after several years. While most patients have few long-term side effects from chemotherapy, their blood counts, kidney function, hearing, and lung function are routinely monitored for signs of chemotherapy toxicities.
Statistics
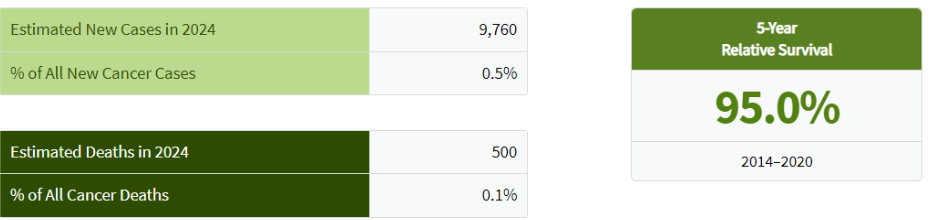
Germ cell tumors (GCTs) are a category of cancers originating from primordial germ cells, the cells destined to become sperm or eggs. These tumors can manifest in the testes as testicular germ cell tumors (TGCTs) or in the ovaries as ovarian germ cell tumors (OGCTs). TGCTs hold the distinction of being the most prevalent cancer among young men, affecting individuals primarily between the ages of 15 and 35. Despite representing a relatively small proportion of all cancers, approximately 0.5%, TGCTs have garnered significant medical attention due to their impact on a young demographic. Remarkably, advancements in treatment modalities have led to a substantial increase in cure rates, from around 25% in the 1970s to nearly 80% in contemporary practice. The highest incidence of TGCTs is observed in regions such as Scandinavia, Germany, and New Zealand.
Figure 10. This table shows the estimated cases for TGCTs in 2024 and the % of all new cancer cases, including estimated deaths and 5-year relative survival. SEER Cancer Statistics Factsheets: Testicular Cancer. National Cancer Institute.
In contrast to TGCTs, ovarian germ cell tumors (OGCTs) are less commonly diagnosed but carry a more severe prognosis. While ovarian cancer overall is the fifth leading cause of cancer-related death among women, contributing to 2.5% of all female cancers, it is essential to note that the majority of ovarian cancers are not of germ cell origin. However, OGCTs constitute a significant proportion of ovarian tumors in young women. The peak incidence of OGCTs occurs in women during their twenties and thirties.
Figure 11. This table shows the estimated cases for OGCTs in 2024 and the % of all new cancer cases, including estimated deaths and 5-year relative survival. SEER Cancer Statistics Factsheets: Ovarian Cancer. National Cancer Institute.
The pediatric population is also susceptible to GCTs. These tumors represent a substantial portion of cancers affecting adolescents, comprising approximately 17% of all cancers in this age group. Age-specific incidence rates follow a bimodal distribution, with an initial peak in early childhood and a subsequent peak in adolescence (Figure 12).
Figure 12. Age-specific incidence of germ cell tumors in males and females ages 0−19 years in the United States, 2015−2019. Source: National Childhood Cancer Registry.
With an estimated 15,780 cases in the Americas and 69,600 globally, the impact of GCTs on children and adolescents is evident. Geographic disparities in the incidence of pediatric GCTs are pronounced, with countries like Japan, Denmark, and Singapore reporting higher rates.
The global burden of germ cell tumors, particularly in young populations, underscores the importance of ongoing research, early detection, and improved treatment strategies. While significant progress has been made in managing these cancers, there remains a need for continued efforts to enhance patient outcomes and reduce the impact of GCTs on individuals and communities worldwide.
Impacts and complications
Gonadal germ cell tumors (GCTs) are a significant concern in the field of oncology, affecting both males and females, predominantly in the testes and ovaries. GCTs have profound impacts on the physical, emotional, and psychological well-being of affected individuals. Physically, these tumors can lead to significant health challenges, including the potential loss of reproductive organs, which directly affects fertility. The aggressive nature of some GCTs may necessitate intensive treatments like chemotherapy, surgery, and radiation, resulting in long-term side effects such as fatigue, pain, and secondary health issues. Emotionally, the diagnosis of a GCT can cause significant distress, leading to anxiety, depression, and concerns about recurrence, impacting the overall quality of life.
Gonadal germ cell tumors (GCTs) are significantly influenced by a combination of genetic, environmental, and physiological risk factors. Cryptorchidism (undescended testis), atrophic testis, hypospadias, and testicular dysgenesis syndrome are prominent conditions linked to an elevated risk of developing these tumors. Additionally, a family history of testicular germ cell tumors (TGCT), hereditary syndromes like Cowden syndrome, and low sperm count are crucial genetic factors that increase susceptibility.
Exogenous exposure to estrogen, particularly during fetal development, has also been identified as a significant environmental risk factor. These risk factors collectively contribute to the complexity of diagnosing and treating GCTs, making it essential for healthcare providers to consider them when developing early detection strategies and personalized treatment plans. Understanding these risk factors aids in improving patient outcomes and overall quality of life post-treatment.
Conclusion
Raising awareness about Germ Cell Tumors (GCTs) is critically important for patients, families, healthcare providers, and the public at large. Numerous methods exist to enhance awareness and safety regarding this disease, including the use of social media platforms, fundraising activities, public events, and educational campaigns. These strategies are highly effective in preventing the spread of GCTs, and regardless of the outcomes, societal support is advantageous for everyone. As a community, we have the opportunity to utilize these methods for the greater good, aiding not just acquaintances and friends but also family members.
References
De Felici, M.; Klinger, F.G.; Campolo, F.; Balistreri, C.R.; Barchi, M.; Dolci, S. To Be or Not to Be a Germ Cell: The Extragonadal Germ Cell Tumor Paradigm. Int. J. Mol. Sci. 2021, 22, 5982.
Altinok I, Ozturk RC, Kahraman UC, Capkin E. Protection of rainbow trout against yersiniosis by lpxD mutant Yersinia ruckeri. Fish Shellfish Immunol. 2016 Aug;55:21-7. doi: 10.1016/j.fsi.2016.04.018. Epub 2016 Apr 16. PMID: 27095175.
Cools, Martine & Drop, S.L.s & Wolffenbuttel, K.P. & Oosterhuis, Wolter & Looijenga, Leendert. (2006). Germ Cell Tumors in the Intersex Gonad: Old Paths, New Directions, Moving Frontiers. Endocrine reviews. 27. 468-84. 10.1210/er.2006-0005.
Steinmacher, Sahra & Sara Yvonne, Brucker & Kölle, Andrina & Kraemer, Bernhard & Schöller, Dorit & Rall, Katharina. (2021). Malignant Germ Cell Tumors and Their Precursor Gonadal Lesions in Patients with XY-DSD: A Case Series and Review of the Literature. International Journal of Environmental Research and Public Health. 18. 5648. 10.3390/ijerph18115648.
Piazza, Mauri & Urbanetz, Almir. (2019). Germ Cell Tumors in Dysgenetic Gonads. Clinics. 74. 10.6061/clinics/2019/e408.
Lin, Xiaokun & Wu, Dazhou & Zheng, Na & Xia, Qiongzhang & Han, Yijiang. (2017). Gonadal germ cell tumors in children: A retrospective review of a 10-year single-center experience. Medicine. 96. e7386. 10.1097/MD.0000000000007386.
Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995 Jun;16(3):271-321. doi: 10.1210/edrv-16-3-271. Erratum in: Endocr Rev 1995 Aug;16(4):546. PMID: 7671849.
Müller J, Skakkebaek NE. Testicular carcinoma in situ in children with the androgen insensitivity (testicular feminisation) syndrome. Br Med J (Clin Res Ed). 1984 May 12;288(6428):1419-20. doi: 10.1136/bmj.288.6428.1419-a. PMID: 6426583; PMCID: PMC1441073.
Houk CP, Lee PA 2005 Intersexed states: diagnosis and management. Endocrinol Metab Clin North Am 34:791– 810, xi.
Grumbach MM, Hughes IA, Conte FA 2003 Disorders of sex differentiation. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KM, eds. Williams textbook of endocrinology. 10th ed. Philadelphia: W.B. Saunders (Elsevier); 842–1002.
Reiner WG, Gearhart JP 2004 Discordant sexual identity in some genetic males with cloacal exstrophy assigned to female sex at birth. N Engl J Med 350:333–341
Money J, Ehrhardt AA 1972 Man and woman, boy and girl. Baltimore: Johns Hopkins University Press
Johns Hopkins Medicine. (n.d.). Germ cell tumors. Sidney Kimmel Comprehensive Cancer Center. https://www.hopkinsmedicine.org/kimmel-cancer-center/cancers-we-treat/pediatric/about-us/germ-cell-tumors
NYU Langone Health. (n.d.). Diagnosis of germ cell tumors in children. https://nyulangone.org/conditions/germ-cell-tumors-in-children/diagnosis
Foster, R. S., & Bihrle, R. (2016). Late relapse of germ cell tumors: Treatment strategies and outcomes. Journal of Oncology Practice, 12(6), 486-490.
Oosterhuis JW, Looijenga LH 2005 Testicular germ-cell tumors in a broader perspective. Nat Rev Cancer 5:210 –222
Cassio A, Cacciari E, D’Errico A, Balsamo A, Grigioni FW, Pascucci MG, Bacci F, Tacconi M, Mancini AM 1990 Incidence of intratubular germ cell neoplasia in androgen insensitivity syndrome. Acta Endocrinol (Copenh) 123:416 – 422
Sinnecker GH, Hiort O, Nitsche EM, Holterhus PM, Kruse K 1997 Functional assessment and clinical classification of androgen sensitivity in patients with mutations of the androgen receptor gene. German Collaborative Intersex Study Group. Eur J Pediatr 156:7–14
Boehmer AL, Brinkmann O, Bruggenwirth H, van Assendelft C, Otten BJ, Verleun-Mooijman MC, Niermeijer MF, Brunner HG, Rouwe CW, Waelkens JJ, Oostdijk W, Kleijer WJ, van der Kwast TH, de Vroede MA, Drop SL 2001 Genotype versus phenotype in families with androgen insensitivity syndrome. J Clin Endocrinol Metab 86:4151– 4160
Ahmed SF, Cheng A, Dovey L, Hawkins JR, Martin H, Rowland J, Shimura N, Tait AD, Hughes IA 2000 Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome. J Clin Endocrinol Metab 85:658 – 665
Chen CP, Chern SR, Wang TY, Wang W, Wang KL, Jeng CJ 1999 Androgen receptor gene mutations in 46,XY females with germ cell tumours. Hum Reprod 14:664 – 670
Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS 1995 Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 16:271–321 55. Chen CP, Chern SR, Wang TY, Wang W, Wang KL,
Sawsan Rashdan et al, Salvage Therapy for Patients With Germ Cell Tumor. JOP 12, 437-443(2016)
Giwercman A, Muller J, Skakkebaek NE 1991 Prevalence of carcinoma in situ and other histopathological abnormalities in testes from 399 men who died suddenly and unexpectedly. J Urol 145: 77– 80
Muller J, Skakkebaek NE 1984 Testicular carcinoma in situ in children with the androgen insensitivity (testicular feminisation) syndrome. Br Med J (Clin Res Ed) 288:1419 –1420
MacLaughlin DT, Donahoe PK 2004 Sex determination and differentiation. N Engl J Med 350:367–378
Fleming A, Vilain E 2005 The endless quest for sex determination genes. Clin Genet 67:15–25
Veitia RA, Salas-Cortes L, Ottolenghi C, Pailhoux E, Cotinot C, Fellous M 2001 Testis determination in mammals: more questions than answers. Mol Cell Endocrinol 179:3–16
Ahmed SF, Hughes IA 2002 The genetics of male undermasculinization. Clin Endocrinol (Oxf) 56:1–18
Zhang P, Feng Z, Cai W, et al. T2-Weighted Image-Based Radiomics Signature for Discriminating Between Seminomas and Nonseminoma. Front Oncol 2019;91330.
Cools M, Stoop H, Kersemaekers AMF, Drop SLS, Wolffenbuttel KP, Bourguignon J-P, Slowikowska-Hilczer J, Kula K, Faratz S, Oosterhuis JW, Looijenga LHJ 2006 Gonadoblastoma arising in undifferentiated gonadal tissue within dysgenetic gonads. J Clin Endocrinol Metab 91:2404 –2413
Trobs RB, Hoepffner W, Buhligen U, Limbach A, Keller E, Schutz A, Horn LC, Kiess W, Bennek J 2004 Video-assisted gonadectomy in children with Ullrich Turner syndrome or 46,XY gonadal dysgenesis. Eur J Pediatr Surg 14:179 –184
Slowikowska-Hilczer J, Szarras-Czapnik M, Kula K 2001 Testicular pathology in 46,XY dysgenetic male pseudohermaphroditism: an approach to pathogenesis of testis cancer. J Androl 22:781–792
Donahoe PK, Crawford JD, Hendren WH 1979 Mixed gonadal dysgenesis, pathogenesis, and management. J Pediatr Surg 14:287–300
Slowikowska-Hilczer J, Romer TE, Kula K 2003 Neoplastic potential of germ cells in relation to disturbances of gonadal organogenesis and changes in karyotype. J Androl 24:270 –278
Krasna IH, Lee ML, Smilow P, Sciorra L, Eierman L 1992 Risk of malignancy in bilateral streak gonads: the role of the Y chromosome. J Pediatr Surg 27:1376 –138
Lenz S, Skakkebaek NE, Hertel NT. Abnormal ultrasonic pattern in contralateral testes in patients with unilateral testicular cancer. World J Urol 1996;14(Suppl 1):S55–S58.
Howitt BE, Berney DM. Tumors of the Testis: Morphologic Features and Molecular Alterations. Surg Pathol Clin 2015;8(4):687–716.
Marko J, Wolfman DJ, Aubin AL, Sesterhenn IA. Testicular Seminoma and Its Mimics: From the Radiologic Pathology Archives. RadioGraphics 2017;37(4):1085–1098.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021;71(1):7–33.
Williamson SR, Delahunt B, Magi-Galluzzi C, et al. The World Health Organization 2016 classification of testicular germ cell tumours: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology 2017;70(3):335–346.
Baraban EG, Cooper K. Pathogenesis of Testicular Germ Cell Neoplasia: A Conceptual Approach. Adv Anat Pathol 2019;26(4):241–245.
Ulbright TM. Recently Described and Clinically Important Entities in Testis Tumors: A Selective Review of Changes Incorporated Into the 2016 Classification of the World Health Organization. Arch Pathol Lab Med 2019;143(6):711–721.
Ulbright TM, Amin MB, Balzer BL. Germ cell tumors. In: World Health Organization classification of tumours of the urinary system and male genital organs. Lyon, France: IARC Press, 2016; 189–226.
Coursey Moreno C, Small WC, Camacho JC, et al. Testicular tumors: what radiologists need to know–differential diagnosis, staging, and management. RadioGraphics 2015;35(2):400–415..
Thomas KL, Jeong D, Montilla-Soler J, Feuerlein S. The role of diagnostic imaging in the primary testicular cancer: initial staging, response assessment and surveillance. Transl Androl Urol 2020;9(Suppl 1):S3–S13.
Cheng L, Albers P, Berney DM, et al. Testicular cancer. Nat Rev Dis Primers 2018;4(1):29.
Liu R, Lei Z, Li A, Jiang Y, Ji J. Differentiation of testicular seminoma and nonseminomatous germ cell tumor on magnetic resonance imaging. Medicine (Baltimore) 2019;98(45):e17937.
Marshall C, Enzerra M, Rahnemai-Azar AA, Ramaiya NH. Serum tumor markers and testicular germ cell tumors: a primer for radiologists. Abdom Radiol (NY) 2019;44(3):1083–1090.
Stephenson A, Eggener SE, Bass EB, et al. Diagnosis and Treatment of Early Stage Testicular Cancer: AUA Guideline. J Urol 2019;202(2):272–281.
Busch J, Seidel C, Zengerling F. Male Extragonadal Germ Cell Tumors of the Adult. Oncol Res Treat 2016;39(3):140–144.
Baroni T, Arato I, Mancuso F, Calafiore R, Luca G. On the Origin of Testicular Germ Cell Tumors: From Gonocytes to Testicular Cancer. Front Endocrinol (Lausanne) 2019;10(343):343.
Looijenga LHJ, Van der Kwast TH, Grignon D, et al. Report From the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers: IV: Current and Future Utilization of Molecular-Genetic Tests for Testicular Germ Cell Tumors. Am J Surg Pathol 2020;44(7):e66–e79.
Elzinga-Tinke JE, Dohle GR, Looijenga LH. Etiology and early pathogenesis of malignant testicular germ cell tumors: towards possibilities for preinvasive diagnosis. Asian J Androl 2015;17(3):381–393.
Nead KT, Mitra N, Weathers B, et al. Lower abdominal and pelvic radiation and testicular germ cell tumor risk. PLoS One 2020;15(11):e0239321.
Revels JW, Wang SS, Gangadhar K, Ali A, Ali AA, Lee JH. Multimodality Radiological Pictorial Review of Testicular Carcinoma: From Initial Staging to Restaging. Res Rep Urol 2020;12(599):613.
Yacoub JH, Oto A, Allen BC, et al. ACR Appropriateness Criteria Staging of Testicular Malignancy. J Am Coll Radiol 2016;13(10):1203–1209.
Wood MJ, Thomas R, Howard SA, Braschi-Amirfarzan M. Imaging of Metastatic Germ Cell Tumors in Male Patients From Initial Diagnosis to Treatment-Related Toxicities: A Primer for Radiologists. AJR Am J Roentgenol 2020;214(1):24–33.
Dogra VS, Gottlieb RH, Oka M, Rubens DJ. Sonography of the scrotum. Radiology 2003;227(1):18–36.
Guideline developed in collaboration with the American College of Radiology; Society for Pediatric Radiology; Society of Radiologists in Ultrasound. AIUM Practice Guideline for the Performance of Scrotal Ultrasound Examinations. J Ultrasound Med 2015;34(8):1–5.
Coret A, Leibovitch I, Heyman Z, Goldwasser B, Itzchak Y. Ultrasonographic evaluation and clinical correlation of intratesticular lesions: a series of 39 cases. Br J Urol 1995;76(2):216–219.
Huang DY, Sidhu PS. Focal testicular lesions: colour Doppler ultrasound, contrast-enhanced ultrasound and tissue elastography as adjuvants to the diagnosis. Br J Radiol 2012;85(Spec No 1):S41–S53.
Schwarze V, Marschner C, Sabel B, et al. Multiparametric ultrasonographic analysis of testicular tumors: a single-center experience in a collective of 49 patients. Scand J Urol 2020;54(3):241–247.
Lerchbaumer MH, Auer TA, Marticorena GS, et al. Diagnostic performance of contrast-enhanced ultrasound (CEUS) in testicular pathologies: Single-center results. Clin Hemorheol Microcirc 2019;73(2):347–357.
Pierorazio PM, Cheaib JG, Tema G, et al. Performance Characteristics of Clinical Staging Modalities for Early Stage Testicular Germ Cell Tumors: A Systematic Review. J Urol 2020;203(5):894–901.
Tsili AC, Bertolotto M, Rocher L, et al. Sonographically indeterminate scrotal masses: how MRI helps in characterization. Diagn Interv Radiol 2018;24(4):225–236.
Rud E, Langberg CW, Baco E, Lauritzen P, Sandbæk G. MRI in the Follow-up of Testicular Cancer: Less is More. Anticancer Res 2019;39(6):2963–2968.
Dotzauer R, Thomas C, Jäger W. The use of F-FDG PET/CT in testicular cancer. Transl Androl Urol 2018;7(5):875–878.
Calabrò D, Telo S, Ambrosini V. PET imaging in testicular tumours. Curr Opin Urol 2020;30(5):665–671.
Winter TC, Kim B, Lowrance WT, Middleton WD. Testicular Microlithiasis: What Should You Recommend? AJR Am J Roentgenol 2016;206(6):1164–1169.
Volokhina YV, Oyoyo UE, Miller JH. Ultrasound demonstration of testicular microlithiasis in pediatric patients: is there an association with testicular germ cell tumors? Pediatr Radiol 2014;44(1):50–55.
Trout AT, Chow J, McNamara ER, et al. Association between Testicular Microlithiasis and Testicular Neoplasia: Large Multicenter Study in a Pediatric Population. Radiology 2017;285(2):576–583.
Heller HT, Oliff MC, Doubilet PM, O’Leary MP, Benson CB. Testicular microlithiasis: prevalence and association with primary testicular neoplasm. J Clin Ultrasound 2014;42(7):423–426.
Richenberg J, Belfield J, Ramchandani P, et al. Testicular microlithiasis imaging and follow-up: guidelines of the ESUR scrotal imaging subcommittee. Eur Radiol 2015;25(2):323–330.
Gupta M, Cheaib JG, Patel HD, et al. Diagnosis and Management of Intratubular Germ Cell Neoplasia In Situ: A Systematic Review. J Urol 2020;204(1):33–41.
Lenz S, Skakkebaek NE, Hertel NT. Abnormal ultrasonic pattern in contralateral testes in patients with unilateral testicular cancer. World J Urol 1996;14(Suppl 1):S55–S58.
Howitt BE, Berney DM. Tumors of the Testis: Morphologic Features and Molecular Alterations. Surg Pathol Clin 2015;8(4):687–716
Marko J, Wolfman DJ, Aubin AL, Sesterhenn IA. Testicular Seminoma and Its Mimics: From the Radiologic Pathology Archives. RadioGraphics 2017;37(4):1085–1098.
Woodward PJ, Sohaey R, O’Donoghue MJ, Green DE. From the archives of the AFIP: tumors and tumorlike lesions of the testis: radiologic-pathologic correlation. RadioGraphics 2002;22(1):189–216.
Sangüesa C, Veiga D, Llavador M, Serrano A. Testicular tumours in children: an approach to diagnosis and management with pathologic correlation. Insights Imaging 2020;11(1):74.
Mittal PK, Abdalla AS, Chatterjee A, et al. Spectrum of Extratesticular and Testicular Pathologic Conditions at Scrotal MR Imaging. RadioGraphics 2018;38(3):806–830.
Rebik K, Wagner JM, Middleton W. Scrotal Ultrasound. Radiol Clin North Am 2019;57(3):635–648.
Sharbidre KG, Lockhart ME. Imaging of scrotal masses. Abdom Radiol (NY) 2020;45(7):2087–2108.
Angulo JC, González J, Rodríguez N, et al. Clinicopathological study of regressed testicular tumors (apparent extragonadal germ cell neoplasms). J Urol 2009;182(5):2303–2310.
Tasu JP, Faye N, Eschwege P, Rocher L, Bléry M. Imaging of burned-out testis tumor: five new cases and review of the literature. J Ultrasound Med 2003;22(5):515–521.
Chang MY, Shin HJ, Kim HG, Kim MJ, Lee MJ. Prepubertal Testicular Teratomas and Epidermoid Cysts: Comparison of Clinical and Sonographic Features. J Ultrasound Med 2015;34(10):1745–1751.
Cho JH, Chang JC, Park BH, Lee JG, Son CH. Sonographic and MR imaging findings of testicular epidermoid cysts. AJR Am J Roentgenol 2002;178(3):743–748.
Magudia K, Menias CO, Bhalla S, Katabathina VS, Craig JW, Hammer MM. Unusual Imaging Findings Associated with Germ Cell Tumors. RadioGraphics 2019;39(4):1019–1035.
Denaro L, Pluchinotta F, Faggin R, et al. What’s growing on? The growing teratoma syndrome. Acta Neurochir (Wien) 2010;152(11):1943–1946.
Uguz S, Yilmaz S, Guragac A, Topuz B, Aydur E. Association of Torsion With Testicular Cancer: A Retrospective Study. Clin Genitourin Cancer 2016;14(1):e55–e57
Kaikani W, Boyle H, Chatte G, et al. Sarcoid-like granulomatosis and testicular germ cell tumor: the ‘Great Imitator’. Oncology 2011;81(5-6):319–324.
Auer T, De Zordo T, Dejaco C, et al. Value of Multiparametric US in the Assessment of Intratesticular Lesions. Radiology 2017;285(2):640–649.
What are Germ Cell Tumors? Germ Cell Tumors - Brigham and Women's Hospital
Muller J, Skakkebaek NE 1984 Testicular carcinoma in situ in children with the androgen insensitivity (testicular feminisation) syndrome. Br Med J (Clin Res Ed) 288:1419 –1420
macLaughlin DT, Donahoe PK 2004 Sex determination and diff
(n.d.). Germ Cell Tumors (GCTs) in Children. Nationwide Children's Hospital.
Fleming A, Vilain E 2005 The endless quest for sex determination genes. Clin Genet 67:15–25
Veitia RA, Salas-Cortes L, Ottolenghi C, Pailhoux E, Cotinot C, Fellous M 2001 Testis determination in mammals: more questions than answers. Mol Cell Endocrinol 179:3–16
ed SF, Hughes IA 2002 The genetics of male undermasculinization. Clin Endocrinol (Oxf) 56:1–18
(n.d.). Steps + Salary Information. How to Become an Oncologist.
Cools M, Stoop H, Kersemaekers AMF, Drop SLS, Wolffenbuttel KP, Bourguignon J-P, Slowikowska-Hilczer J, Kula K, Faratz S, Oosterhuis JW, Looijenga LHJ 2006 Gonadoblastoma arising in undifferentiated gonadal tissue within dysgenetic gonads. J Clin Endocrinol Metab 91:2404 –2413
Trobs RB, Hoepffner W, Buhligen U, Limbach A, Keller E, Schutz A, Horn LC, Kiess W, Bennek J 2004 Video-assisted gonadectomy in children with Ullrich Turner syndrome or 46,XY gonadal dysgene
lowikowska-Hilczer J, Szarras-Czapnik M, Kula K 2001 Testicular pathology in 46,XY dysgenetic male pseudohermaphroditism: an approach to pathogenesis of testis cancer. J Androl 22:781–792
(2022, February 21). All About Surgical Oncology at TSC. The Surgical Clinic.
Nishi MY, Domenice S, Medeiros MA, Mendonca BB, Billerbeck AE 2002 Detection of Y-specific sequences in 122 patients with Turner syndrome: nested PCR is not a reliable method. Am J Med Genet 107:299 –305
(2022, February 21). All About Surgical Oncology at TSC. The Surgical Clinic.
Donahoe PK, Crawford JD, Hendren WH 1979 Mixed gonadal dysgenesis, pathogenesis, and management. J Pediatr Surg 14:287–300
Slowikowska-Hilczer J, Romer TE, Kula K 2003 Neoplastic potential of germ cells in relation to disturbances of gonadal organogenesis and changes in karyotype. J Androl 24:270 –278
(2024, June 25). Surgical Oncologist Salary (August 2024). Zippia.
Cheng L, Thomas A, Roth LM, Zheng W, Michael H, Karim FW 2004 OCT4: a novel biomarker for dysgerminoma of the ovary. Am J Surg Pathol 28:1341–1346
Cheng L 2004 Establishing a germ cell origin for metastatic tumors using OCT4 immunohistochemistry. Cancer 101:2006 –2010
(n.d.). Article title How to Become a Gynecologist. HospitalCareers.com.
Krasna IH, Lee ML, Smilow P, Sciorra L, Eierman L 1992 Risk of malignancy in bilateral streak gonads: the role of the Y chromosome. J Pediatr Surg 27:1376 –1380
(2018, September 29). What Is a Urologist? What They Do, Procedures, and More. Healthline.
(2023, December 4). Germ cell ovarian tumours. Ovarian Cancer | Cancer Research UK.
Horn LC, Limbach A, Hoepffner W, Trobs RB, Keller E, Froster UG, Richter CE, Jakubiczka S 2005 Histologic analysis of gonadal tissue in patients with Ullrich-Turner syndrome and derivative Y chromosomes. Pediatr Dev Pathol 8:197–203
Muller J, Skakkebaek NE, Ritzen M, Ploen L, Petersen KE 1985 Carcinoma in situ of the testis in children with 45,X/46,XY gonadal dysgenesis. J Pediatr 106:431– 436
Medlej R, Lobaccaro JM, Berta P, Belon C, Leheup B, Toublanc JE, Weill J, Chevalier C, Dumas R, Sultan C 1992 Screening for Y-derived sex determining gene SRY in 40 patients with Turner syndrome. J Clin Endocrinol Metab 75:1289 –1292
Lopez M, Canto P, Aguinaga M, Torres L, Cervantes A, Alfaro G, Mendez JP, Kofman-Alfaro S 1998 Frequency of Y chromosomal material in Mexican patients with Ullrich-Turner syndrome. Am J Med Genet 76:120 –124
(n.d.). Urologist Salary. Salary.com.
(n.d.). Radiation Oncologist. Cleveland Clinic
Osipova GR, Karmanov ME, Kozlova SI, Evgrafov OV 1998 PCR detection of Y-specific sequences in patients with Ullrich-Turner syndrome: clinical implications and limitations. Am J Med Genet 76:283–287
Mendes JR, Strufaldi MW, Delcelo R, Moises RC, Vieira JG, Kasamatsu TS, Galera MF, Andrade JA, Verreschi IT 1999 Ychromosome identification by PCR and gonadal histopathology in Turner’s syndrome without overt Y-mosaicism. Clin Endocrinol (Oxf) 50:19 –26
(n.d.). Radiation Oncology - Conditions treated - Mayo Clinic. Mayo Clinic.
Gravholt CH, Fedder J, Naeraa RW, Muller J 2000 Occurrence of gonadoblastoma in females with Turner syndrome and Y chromosome material: a population study. J Clin Endocrinol Metab 85: 3199 –3202
(n.d.). Childhood Central Nervous System Germ Cell Tumors Treatment. Childhood Central Nervous System Germ Cell
Fernandez-Garcia R, Garcia-Doval S, Costoya S, Pasaro E 2000 Analysis of sex chromosome aneuploidy in 41 patients with Turner syndrome: a study of ‘hidden’ mosaicism. Clin Genet 58:201–208
Alvarez-Nava F, Soto M, Sanchez MA, Fernandez E, Lanes R 2003 Molecular analysis in Turner syndrome. J Pediatr 142:336 –340
(n.d.). What Is the Average Pathologist Salary by State in 2024? Zippia.
Joki-Erkkilä MM, Karikoski R, Rantala I, Lenko HL, Visakorpi T, Heinonen PK 2002 Gonadoblastoma and dysgerminoma associated with XY gonadal dysgenesis in an adolescent with chronic renal failure: a case of Frasier syndrome. J Pediatr Adolesc Gynecol 15:145–149
Pelletier J, Bruening W, Kashtan CE, Mauer SM, Manivel JC, Striegel JE, Houghton DC, Junien C, Habib R, Fouser L, Fine RN, Silverman BL, Haber DA, Housman D 1991 Germline mutations in the Wilms’ tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell 67:437– 447
(n.d.). Radiation Oncologist Salary. Salary.com.
Pena-Alonso R, Nieto K, Alvarez R, Palma I, Najera N, Erana L, Dorantes LM, Kofman-Alfaro S, Queipo G 2005 Distribution of Y-chromosome-bearing cells in gonadoblastoma and dysgenetic testis in 45,X/46,XY infants. Mod Pathol 18:439 – 445
(2024, July 22). What Is a Pediatric Oncologist? UCLA Med School
de Jong J, Stoop H, Dohle GR, Bangma CH, Kliffen M, van Esser JW, van den Bent M, Kros JM, Oosterhuis JW, Looijenga LH 2005 Diagnostic value of OCT3/4 for pre-invasive and invasive testicular germ cell tumours. J Pathol 206:242–249
(n.d.). Salary: Pediatric Oncologist (October, 2023) United States. ZipRecruiter.
Kersemaekers AM, Honecker F, Stoop H, Cools M, Molier M, Wolffenbuttel K, Bokemeyer C, Li Y, Lau YF, Oosterhuis JW, Looijenga LH 2005 Identification of germ cells at risk for neoplastic transformation in gonadoblastoma: an immunohistochemical study for OCT3/4 and TSPY. Hum Pathol 36:512–521
(n.d.). Germ Cell Tumors in Children. Duke Health.
Gidekel S, Pizov G, Bergman Y, Pikarsky E 2003 Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell 4:361–370
Jones TD, Ulbright TM, Eble JN, Cheng L 2004 OCT4: a sensitive and specific biomarker for intratubular germ cell neoplasia of the testis. Clin Cancer Res 10:8544 – 8547
Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L 2004 OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol 28: 935–940
Poynter, J. N., Fonstad, R., Tolar, J., Spector, L. G., & Ross, J. A. (2014). Incidence of intracranial germ cell tumors by race in the United States, 1992–2010. Journal of Neuro-Oncology, 120(2), 381.
McHugh, D. J., Gleeson, J. P., & Feldman, D. R. (2024). Testicular cancer in 2023: Current status and recent progress. CA: A Cancer Journal for Clinicians, 74(2), 167-186.
Lew, C., Liu, H., Hou, J., Huang, T., & Yeh, T. (2022). Pediatric Extracranial Germ Cell Tumors: Review of Clinics and Perspectives in Application of Autologous Stem Cell Transplantation. Cancers, 15(7), 1998.
Bhuta, R., Shah, R., Gell, J. J., Poynter, J. N., Bagrodia, A., Dicken, B. J., Pashankar, F., Frazier, A. L., & Shaikh, F. Children's Oncology Group's 2023 blueprint for research: Germ cell tumors. Pediatric Blood & Cancer, 70, e30562.
(n.d.). National Childhood Cancer Registry Explorer (NCCR*Explorer). National Childhood Cancer Registry Explorer (NCCR*Explorer).
(n.d.). Malignant Ovarian Germ Cell Tumor. Malignant Ovarian Germ Cell Tumor - an Overview | ScienceDirect Topics.
Saliyeva S, Boranbayeva R, Konoplya N, Bulegenova M, Blau O, Belousov V, Granica J, Mukushkina D, Altynbayeva G. Pediatric Extracranial Germ Cell Tumors: Expression of microRNA. J Pediatr Hematol Oncol. 2023 Mar 1;45(2):e174-e179. doi: 10.1097/MPH.0000000000002495. Epub 2022 Jun 7. PMID: 35700382.










Comments